Rare Earth Elements: The Essential Catalysts of Modern Industry
 If oil is the lifeblood of industry, then rare earths are its vitamins. Rare earths are a valuable strategic resource used in cutting-edge science, technology, and the military, and are considered the “mother of new materials.”
If oil is the lifeblood of industry, then rare earths are its vitamins. Rare earths are a valuable strategic resource used in cutting-edge science, technology, and the military, and are considered the “mother of new materials.”
However, “rare earths are not earths.” Rare earth is the abbreviation for a group of metals. Rare Earth Elements (REEs) have been discovered since the late 18th century. Scientists discover new uses for rare earths almost every three to five years, and one out of every six inventions relies on rare earths.
Rare earths are a valuable strategic resource, known as the “industrial MSG” and the “mother of new materials.” Functional materials such as rare-earth permanent magnets, luminescent materials, hydrogen storage materials, and catalysts are essential for high-tech industries, advanced equipment manufacturing, new energy, and emerging industries.
According to the 2015 data from the U.S. Geological Survey, the world’s rare earth reserves are approximately 130 million tons (measured in rare earth oxides (REOs)). Of these, China holds 55 million tons, Brazil 22 million tons, the United States 13 million tons, Australia 2.1 million tons, India 3.1 million tons, Malaysia 30,000 tons, and other countries 41 million tons.
List of uses of 17 rare earth elements
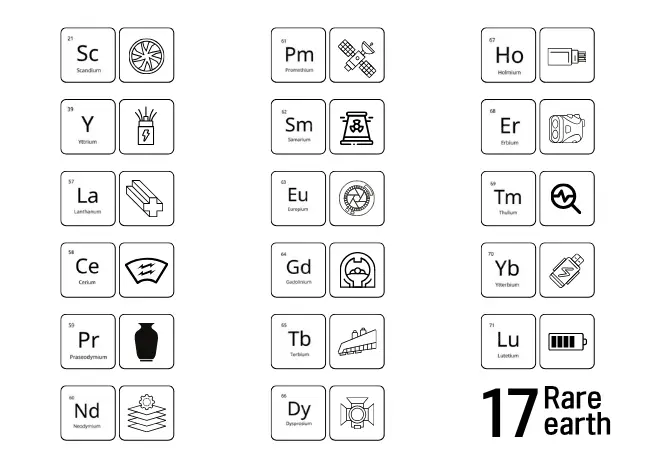
- Lanthanum is the main component of the alloy materials and agricultural films.
- Cerium is the leading element in the automotive glass industry.
- Praseodymium is the element that is abundantly used in ceramic pigments.
- Neodymium finds wide applications in the manufacturing of aerospace materials.
- Promethium is the source of auxiliary energy for the satellites.
- Samarium is the element that is utilized in nuclear reactors.
- Europium is a component of lens manufacturing and liquid crystal display screens.
- Gadolinium acts as a contrast agent in Medical MRIs.
- Terbium is used in the aircraft wing regulators.
- Erbium is one of the parts employed for military laser rangefinders.
- Dysprosium is the element that is the lighting source for film and printing.
- Holmium is a component of optical communication devices.
- Thulium makes the clinical diagnosis and treatment of tumours possible.
- Ytterbium is an additive in computer memory devices.
- Lutetium is used in energy battery technology.
- Yttrium is used in electrical wiring and aircraft load-bearing components.
- Scandium is often used in alloy manufacturing.
Lanthanum (La)

Lanthanum has a wide range of applications, such as piezoelectric materials, thermoelectric materials, magnetoresistive materials, luminescent materials, hydrogen storage materials, optical glass, laser materials, various alloy materials, etc. Lanthanum is also used in the preparation of catalysts for many organic chemical products. Lanthanum is also used in photoconversion agricultural films. Scientists have named Lanthanum “super calcium” for its effect on crops.
Cerium (Ce)

(1) As a glass additive, Cerium can absorb ultraviolet and infrared rays and is now widely used in automotive glass. It protects against ultraviolet rays and reduces the temperature inside the car, thereby saving electricity for air conditioning. Since 1997, all Japanese automobile glass has been added with cerium oxide. In 1996, at least 2,000 tons of cerium oxide were used for automobile glass, and the United States used about 1,000 tons.
(2) Cerium is currently used in automobile exhaust purification catalysts, which can effectively prevent large amounts of automobile exhaust from being discharged into the air. The United States’ consumption in this area accounts for one-third of the total rare earth consumption.
(3) Cerium sulfide is safer for the environment and humans and can replace metals such as lead and cadmium in pigments. They not only help to colour plastics but are also good for coatings, inks, and the paper industry. The French Rhone-Poulenc Company is the market leader at present.
(4) Ce: LiSAF laser system is a solid-state laser developed by the United States. It can detect biological weapons by monitoring tryptophan concentration, and can also be used in medicine. Cerium has a wide range of applications and is contained in almost all rare earth applications, such as polishing powder, hydrogen storage materials, thermoelectric materials, cerium tungsten electrodes, ceramic capacitors, piezoelectric ceramics, cerium silicon carbide abrasives, fuel cell raw materials, gasoline catalysts, certain permanent magnet materials, various alloy steels, and nonferrous metals.
Praseodymium (Pr)

“Praseodymium-neodymium” means “twins” in Greek. About 40 years later, in 1885, when the gas lamp shade was invented, the Austrian Welsbach successfully separated two elements from “praseodymium-neodymium”, one named “neodymium” and the other named “praseodymium”. These “twins” were separated, and praseodymium had a broad world to display its talents.
Praseodymium, a rare earth element, is the main ingredient in glass, ceramics, and magnetic materials.
(1) It is used to make permanent magnets. The use of cheap praseodymium-neodymium metal instead of pure neodymium metal to manufacture permanent magnet materials has significantly improved its antioxidant properties and mechanical properties, and can be processed into magnets of various shapes. It is widely used in various electronic devices and motors.
(2) Petroleum catalytic cracking is one of the uses. The catalyst for cracking petroleum, which was made by the addition of praseodymium and neodymium-rich substances in Y-type zeolite molecular sieve, can make the catalyst better in terms of activity, selectivity, and stability and bring the catalyst to higher catalytic performance.
(3) Praseodymium is also a good choice for abrasive polishing. Besides that, praseodymium is getting more and more used in the area of optical fibres. With the birth of praseodymium, neodymium also came into being. The arrival of neodymium has activated the rare earth field, played an important role in the rare earth field, and influenced the rare earth market.
Neodymium (Nd)

Promethium (Pm)

(1) It can be used as a heat source. It provides auxiliary energy for vacuum detection and artificial satellites.
(2) Pm147 emits low-energy beta rays and makes promethium batteries. It is used as a power source for missile guidance instruments and clocks. This type of battery is small and can be used continuously for several years. Promethium is also used in portable X-ray instruments, the preparation of phosphors, thickness measurement, and navigation lights.
Samarium (Sm)

Samarium is light yellow in colour and is the raw material for samarium-cobalt permanent magnets, the earliest rare earth magnets to be used industrially. These permanent magnets come in two types: the SmCo5 series and the Sm2Co17 series. The SmCo5 series was invented in the early 1970s, followed by the Sm2Co17 series later. Currently, demand for the latter type is predominant. The purity of the samarium oxide used in samarium-cobalt magnets does not need to be very high; for cost reasons, products with a purity of around 95% are primarily used. Samarium oxide is used in ceramic capacitors and catalysts. Furthermore, samarium has nuclear properties and can be used as a structural, shielding, and control material in nuclear reactors, enabling the safe utilization of the enormous energy generated by nuclear fission.
Europium (Eu)

Gadolinium (Gd). In 1880, G. de Marignac of Switzerland separated “samarium” into two elements, one of which was confirmed to be samarium by Sorit, and the other was confirmed by Boisbaudret’s research. In 1886, Marignac named this new element gadolinium in honour of the discoverer of yttrium, the Dutch chemist Gadolin, a pioneer in rare earth research. Gadolinium will play an important role in modern technological innovation. Its main uses are:
(1) Its water-soluble paramagnetic complex can improve the human body’s nuclear magnetic resonance (NMR) imaging signal in medicine.
(2) Its sulfur oxide can be used as a matrix grid for oscilloscopes with special brightness and X-ray fluorescent screens.
(3) Gadolinium in gadolinium gallium garnet is an ideal single substrate for bubble memory storage.
(4) It can be used as a solid magnetic refrigeration medium when there is no Camot cycle limit.
(5) Used as an inhibitor to control the chain reaction level in nuclear power plants to ensure the safety of nuclear reactions.
(6) Used as an additive in samarium cobalt magnets to ensure that their performance does not change with temperature.
Gadolinium (Gd)

Its main uses are:
(1) Its water-soluble paramagnetic complex can improve the human body’s nuclear magnetic resonance (NMR) imaging signal in medicine.
(2) Its sulfur oxide can be used as a matrix grid for oscilloscopes with special brightness and X-ray fluorescent screens.
(3) Gadolinium in gadolinium gallium garnet is an ideal single substrate for bubble memory storage.
(4) It can be used as a solid magnetic refrigeration medium when there is no Camot cycle limit.
(5) Used as an inhibitor to control the chain reaction level in nuclear power plants to ensure the safety of nuclear reactions.
(6) Used as an additive in samarium cobalt magnets to ensure that their performance does not change with temperature.
Terbium (Tb)

The main application areas are:
(1) Phosphor used as an activator for green powder in tri-colour phosphors, terbium-activated phosphate matrix, terbium-activated silicate matrix, and terbium-activated cerium magnesium aluminate matrix, which all emit green light in the excited state.
(2) Over the last few years, terbium-based magneto-optical materials have reached the point of large-scale production. Tb-Fe amorphous thin film-based magneto-optical discs are nowadays utilized as storage media of computers, and the storage capacity is raised by 10-15 times.
(3) Magneto-optical glass. Faraday rotator glass containing terbium is a key material for manufacturing rotators, isolators, and circulators, and it is widely used in laser technology. In particular, the development of the magnetostrictive alloy terbium-dysprosium iron (TerFenol) has opened up new applications for terbium. Discovered in the 1970s, TerFenol is half terbium and dysprosium, sometimes with holmium added, and the remainder is iron. First developed at the Ames Laboratory in Iowa, USA, TerFenol exhibits a greater dimensional change than typical magnetic materials when exposed to a magnetic field. This change enables precise mechanical motion. TerFenol was initially mainly utilized in sonar but has now been extensively used in various fields of technology, such as fuel injection systems, liquid valve control, and micro-positioning, as well as in mechanical actuators, mechanisms, and wing adjusters for aircraft and space telescopes.
Dysprosium (Dy)

The main uses of dysprosium are:
(1) As an additive for NdFeB permanent magnets. Adding about 2% to 3% of dysprosium to such magnets can increase their coercivity. In the past, the demand for dysprosium was not large, but with the increase in demand for NdFeB magnets, it has become a necessary additive element. The grade must be around 95% to 99.9%, and the demand is also increasing rapidly.
(2) Dysprosium is used as a phosphor activator. Trivalent dysprosium is a promising activating ion for single-luminescence-centre trichromatic luminescent materials. It mainly consists of two emission bands, one for yellow light emission and the other for blue light emission. Dysprosium-doped luminescent materials can be used as trichromatic phosphors.
(3) Dysprosium is an essential metal raw material for the preparation of the giant magnetostrictive alloy terbium dysprosium iron (Terfenol) alloy, which can enable the precise movement of some mechanical systems.
(4) Dysprosium metal can be used as a magneto-optical storage material with high recording speed and reading sensitivity.
(5) It is used in the preparation of dysprosium lamps. The working substance used in dysprosium lamps is dysprosium iodide. This lamp has the advantages of high brightness, good colour, high colour temperature, small size, and stable arc. It has been used as a lighting source for movies, printing, etc.
(6) Because dysprosium has the characteristic of a large neutron capture cross-section, it is used in the atomic energy industry to measure neutron energy spectrum or as a neutron absorber.
Holmium (Ho)

The application field of holmium still needs to be further developed, and the amount used is not very large. Recently, Baotou Steel Rare Earth Research Institute used high-temperature and high-vacuum distillation purification technology to develop high-purity metal holmium with very low non-rare earth impurity content, Ho/ΣRE>99.9%.
The main uses of holmium at present are:
(1) It was used as an additive for metal halide lamps. Metal halide lamps refer to the gas discharge lamps that are based on high-pressure mercury lamps. Its feature is that the bulb is filled with different rare-earth halides. At present, mainly rare earth iodides are used, which, during the gas discharge, emit various spectral line colours. The light source of holmium lamps is holmium iodide, which can produce a higher concentration of metal atoms in the arc zone, thus greatly increasing the radiation efficiency.
(2) Holmium is an element that may be utilized as an additive for either yttrium iron or yttrium aluminium garnet.
(3) Holmium-doped yttrium aluminium garnet (YAG) is a material that can produce laser light at a wavelength of 2μm. Human tissue has a very high absorption efficiency for 2μm laser light, which is almost three orders of magnitude higher than HD: YAG. Suppose we use Ho: YAG lasers for medical surgery. A holmium-free beam generated by holmium crystals can get rid of fat without producing too much heat, thus it is possible to minimize the thermal damage to healthy tissues.
(4) Holmium in very small quantities can be additionally incorporated in the magnetostrictive alloy Terfenol-D to lower the external field needed that corresponds to the alloy’s saturation magnetization.
(5) Holmium-doped optical fibres are suitable for manufacturing various optoelectronic components such as optical fibre lasers, optical fibre amplifiers, optical fibre sensors, and optical communication devices that will be more significant in today’s rapidly growing fibre optic communication.
Erbium (Er)

(1) The light emission of Er3+ at 1550nm is of special significance because this wavelength is exactly at the lowest loss of optical fibre in fibre optic communication, after being excited by light of wavelengths of 980nm and 1480nm, erbium ions (Er3+) transition from the ground state 4I15/2 to the high-energy state 4I13/2. When the Er3+ in the high-energy state transitions back to the ground state, it emits light of 1550nm wavelength. Quartz optical fibre can transmit light of various wavelengths, but the light attenuation rate of different wavelengths differs. The light in the 1550nm band has the lowest light attenuation rate (0.15dB/km) when transmitted in quartz optical fibre, which is almost the lower limit of attenuation rate.
(2) In addition, erbium-doped laser crystals and their output 1730nm and 1550nm lasers are safe for human eyes, have good atmospheric transmission performance, strong ability to penetrate battlefield smoke, have good confidentiality, are not easily detected by the enemy, and have high contrast when illuminating military targets. They have been made into portable laser rangefinders that are safe for human eyes for military use.
(3) Er3+ can be added to glass to make rare-earth glass materials, which are currently the solid laser materials with the largest output pulse energy and the highest output power.
(4) Er3+ can also be used as an active ion for rare-earth upconversion laser materials.
(5) Erbium can also be used for the decolourization and colouring of eyeglass lenses, glass, and crystallized glass.
Thulium (Tm)

The main uses of thulium are as follows: (1) Thulium is used as a radiation source for portable medical X-ray machines. After irradiation in a nuclear reactor, thulium produces an isotope that can emit X-rays. It can be used to make portable blood irradiators. This irradiator can convert thulium-169 into thulium-170 under the action of high-pressure neutron beams, emitting X-rays to irradiate the blood and reduce the number of white blood cells. These white blood cells cause organ transplant rejection, thereby reducing early organ rejection.
(2) Thulium may be employed clinically for diagnosing and treating neoplasms as it exhibits a higher hematopoietic affinity for tumour tissue. The heavy rare earths possess a greater affinity than the light rare earths, and thulium is at the top of the list of the highest affinities.
(3) Thulium is used as an activator, LaOBr: Br (blue), in the phosphor used in X-ray intensifying screens to enhance optical sensitivity, thereby reducing the exposure and harm of X-rays to humans. Compared with the previous calcium tungstate intensifying screens, it can reduce the X-ray dose by 50%, which has important practical significance in medical applications.
(4) Thulium can also be used as an additive in new lighting sources such as metal halide lamps.
(5) Tm3+ can be added to glass to make rare-earth glass laser materials, currently the solid laser materials with the largest output pulse volume and the highest output power. Tm3+ can also be an activating ion for rare-earth up-conversion laser materials.
Ytterbium (Yb)

The main uses of Ytterbium are:
(1) as a heat shield coating material. Ytterbium can be very effective in improving the corrosion resistance of the electroplated zinc layer, and the coating with the addition of Ytterbium is of finer grain and more uniform and compact than the one without the element.
(2) as a magnetostrictive material. This material has the property of supermagnetostriction, that is, expansion in a magnetic field. The alloy mainly comprises ytterbium/ferrite alloy and dysprosium/ferrite alloy, and a certain proportion of manganese is added to produce super magnetostriction.
(3) Ytterbium elements are used for measuring pressure. Experiments have shown that ytterbium elements have high sensitivity within the calibrated pressure range, and at the same time have opened up a new path for applying Ytterbium in pressure measurement.
(4) Resin-based fillings for molar cavities, replacing the previously commonly used silver-mercury alloy.
(5) Japanese researchers have completed the preparation of ytterbium-doped gadolinium gallium garnet embedded circuit waveguide lasers, which is of great significance to the further development of laser technology. In addition, Ytterbium is also used as a phosphor activator, radio ceramics, computer memory element (bubble) additive, glass fibre flux, and optical glass additive.
Lutetium (Lu)

(1) Manufacturing of some special alloys. For instance, neutron activation analysis can be done with a lutetium-aluminium alloy.
(2) Stable lutetium nuclides catalyze petroleum cracking, alkylation, hydrogenation, and polymerization reactions.
(3) Adding elements to yttrium iron or aluminium garnet to improve certain properties.
(4) Raw materials for magnetic bubble storage.
(5) A composite functional crystal, lutetium-doped aluminium tetraborate (Yttrium-Neodymium), belongs to the technical field of salt solution cooling crystal growth. Experiments have shown that lutetium-doped NYAB crystals are superior to NYAB crystals in optical uniformity and laser performance.
(6) Research by relevant foreign departments has found that lutetium has potential uses in electrochromic displays and low-dimensional molecular semiconductors.
In addition, lutetium is also used in energy battery technology and as an activator for phosphors.
Yttrium (Y)

(1) An additive for steel and nonferrous alloys. FeCr alloys typically contain 0.5-4% yttrium, which can improve these stainless steels’ oxidation resistance and ductility. The addition of an appropriate amount of yttrium-rich mixed rare earth to the MB26 alloy significantly enhances the comprehensive performance of the alloy, and it is capable of substituting for some medium-strength aluminium alloys to use as aircraft load-bearing components. A small amount of yttrium-rich rare earth added to the Al-Zr alloy increases its conductivity. Most domestic wire factories have adopted this alloy. Yttrium addition in copper alloy leads to both conductivity and mechanical strength improvement.
(2) Engine components can be developed using silicon nitride ceramic materials containing 6% yttrium and 2% aluminium.
(3) Use a 400-watt neodymium yttrium aluminium garnet laser beam to perform mechanical processing such as drilling, cutting, and welding on large components.
(4) Electron microscope fluorescent screens composed of Y-Al garnet single crystals have high fluorescence brightness, low absorption of scattered light, and good resistance to high temperature and mechanical wear.
(5) High-yttrium structural alloys containing up to 90% yttrium can be used in aviation and other applications requiring low density and high melting point.
(6) Yttrium-doped SrZrO3 high-temperature proton conductive materials, which are currently attracting much attention, are of great significance for the production of fuel cells, electrolytic cells, and gas sensors requiring high hydrogen solubility. In addition, yttrium is also used in high-temperature resistant spray materials, diluents for nuclear reactor fuel, additives for permanent magnet materials, and as a getter in the electronics industry.
Scandium (Sc)

Compared to yttrium and the lanthanides, scandium has a much smaller ionic radius, and its hydroxide is much weaker in alkalinity. Therefore, when scandium and rare earth elements are mixed and treated with ammonia (or very dilute alkali), scandium will precipitate first. Therefore, it can be relatively easily separated from the rare earth elements using the “fractional precipitation” method. Another method is to use the polarisation decomposition of nitrates for separation. Since scandium nitrate is the easiest to decompose, the purpose of the separation can be achieved. Scandium metal can be obtained by electrolysis.
When metallurgical scandium is being processed, three substances, ScCl3, KCl, and LiCl, are mixed and electrolyzed using molten zinc as the cathode. Scandium precipitates on the zinc electrode, and then the zinc is evaporated, thus only scandium metal remains.
Additionally, scandium is very easy to recover during the ore processing to get the uranium, thorium, and lanthanide elements.
It is not only about uranium, thorium, and lanthanide elements, but also the recovery of associated scandium from tungsten and tin ores, which are considered an important source of scandium. Scandium is mostly in a trivalent state in compounds and is very susceptible to oxidation in the form of Sc2O3 by oxygen in air; the lustre of the metal disappears, and the colour changes to dark grey.
Besides that, the main uses of scandium are:
(1) Scandium can react with hot water to release hydrogen and is also easily soluble in acid. It is a strong reducing agent.
(2) Scandium oxides and hydroxides are only alkaline, but their salt ash is almost insoluble in water. Scandium chloride is a white crystal that is easily soluble in water and can be deliquesced in air.
(3) In the metallurgical industry, scandium is often used to make alloys (alloy additives) to improve the alloy’s strength, hardness, heat resistance, and performance. For example, adding a small amount of scandium to molten iron can significantly improve the properties of cast iron, and adding a small amount of scandium to aluminium can improve its strength and heat resistance.
(4) Scandium is an element that finds numerous applications in the semiconductor industry. One such example is the use of scandium sulfite in the semiconductors that have become very popular among various nations; besides this, scandium-containing ferrites are still promising computer cores.
(5) In the chemical industry, scandium compounds are used as alcohol dehydrogenation and dehydration agents, and as efficient catalysts in ethylene production and chlorine production using waste hydrochloric acid.
(6) Special glasses containing scandium can be manufactured in the glass industry.
(7) In the electric light industry, scandium-sodium lamps, made of scandium and sodium, have the advantages of high efficiency and positive light colour.
(8) Scandium exists in nature in the form of 45Sc. In addition, scandium has nine radioactive isotopes, namely 40-44Sc and 46-49Sc. Among them, 46Sc has been used as a tracer in the chemical industry, metallurgy, and oceanography.

I'm dedicated to popular science writing about magnets. My articles mainly focus on their principles, applications, and industry anecdotes. Our goal is to provide readers with valuable information, helping everyone better understand the charm and significance of magnets. At the same time, we're eager to hear your opinions on magnet-related needs. Feel free to follow and engage with us as we explore the endless possibilities of magnets together!



